The CardioPulmonary Laboratory for Experimental and Applied Physiology
Specializing in human integrative physiology research
Glen E. Foster, Ph.D.
Professor
School of Health & Exercise Scienes, The University of British Columbia
Biography
As an integrative human physiologist, my research aims to understand the complex interplay between cardiovascular and respiratory reflexes in the maintenance of homeostasis under environmental stresses and in disease. My research team is constantly developing novel tools, analyses, and technological approaches that permit our research questions to be answered from unique angles. We are continuously learning and adapting our skill set to remain on the cutting edge of innovation and discovery.
- Cardiovascular & Respiratory Control
- Blood flow measurement and regulation
- Autonomic Nervous System
PhD in Cardiovascular and Respiratory Sciences, 2009
University of Calgary
MSc in Kinesiology, 2004
University of British Columbia
BHK in Human Kinetics, 2002
University of British Columbia
Mission & Vision
Mission
The Cardiopulmonary Laboratory for Experimental and Applied Physiology, through discovery and innovation, aims to advance our fundamental understanding of how the cardiovascular, respiratory, and autonomic systems function in concert to support human life in a dynamic environment. Our training environment supports and promotes the development of independent researchers who have the freedom to achieve the highest level of skill proficiency and creativity in their research.
Vision
We envision a world in which the complex interplay between human physiological systems, including the cardiovascular, respiratory, and autonomic nervous systems, are better understood permitting strategies to optimize human health and treat disease.
Research Projects
Featured Publications
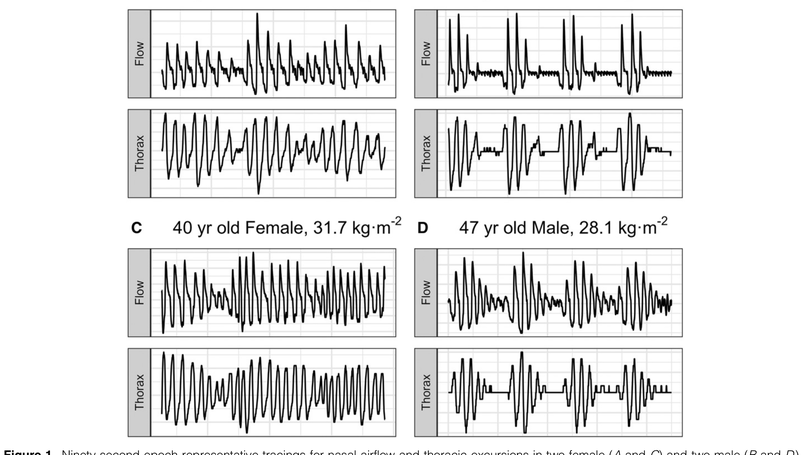
Rationale: Central sleep apnea (CSA) is pervasive during sleep at high altitude, disproportionately impacting men and associated with increased peripheral chemosensitivity. Objectives: We aimed to assess whether biological sex affects loop gain (LGn) and CSA severity during sleep over 9-10 days of acclimatization to 3,800 m. We hypothesized that CSA severity would worsen with acclimatization in men but not in women because of greater increases in LGn in men. Methods: Sleep studies were collected from 20 (12 male) healthy participants at low altitude (1,130 m, baseline) and after ascent to (nights 2/3, acute) and residence at high altitude (nights 9/10, prolonged). CSA severity was quantified as the respiratory event index (REI) as a surrogate of the apnea-hypopnea index. LGn, a measure of ventilatory control instability, was quantified using a ventilatory control model fit to nasal flow. Linear mixed models evaluated effects of time at altitude and sex on respiratory event index and LGn. Data are presented as contrast means with 95% confidence intervals. Results: REI was comparable between men and women at acute altitude (4.1 [-9.3, 17.5] events/h; P = 0.54) but significantly greater in men at prolonged altitude (23.7 [10.3, 37.1] events/h; P = 0.0008). Men had greater LGn than did women for acute (0.08 [0.001, 0.15]; P = 0.047) and prolonged (0.17 [0.10, 0.25]; P < 0.0001) altitude. The change in REI per change in LGn was significantly greater in men than in women (107 ± 46 events/h/LGn; P = 0.02). Conclusions: The LGn response to high altitude differed between sexes and contributed to worsening of CSA over time in men but not in women. This sex difference in acclimatization appears to protect females from high altitude-related CSA. These data provide fundamental sex-specific physiological insight into high-altitude acclimatization in healthy individuals and may help to inform sex differences in sleep-disordered breathing pathogenesis in patients with cardiorespiratory disease.
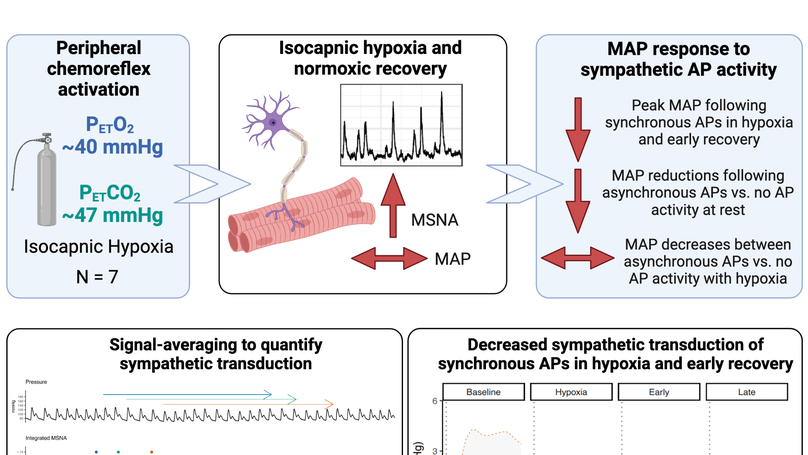
Key points: Acute isocapnic hypoxia elicits lasting sympathoexcitation that does not correspond to parallel changes in vascular tone, suggesting blunted sympathetic transduction. Signal-averaging techniques track the magnitude and temporal cardiovascular responses following integrated muscle sympathetic nerve activity (MSNA) burst and nonburst cardiac cycles; however, this does not fully characterize the effects of sympathetic action potential (AP) activity on blood pressure control. We show that hypoxia blunts the sympathetic transduction of mean arterial pressure (MAP) following synchronous APs that form integrated MSNA bursts and that sympathetic transduction of MAP remains attenuated into early recovery. At rest, asynchronous APs attenuate the reduction in MAP compared to cardiac cycles following no AP activity, thus asynchronous sympathetic APs appear to contribute to the neural regulation of blood pressure. The results advance our understanding of sympathetic transduction of arterial pressure during and following exposure to acute isocapnic hypoxia in humans. Abstract: Post-hypoxia sympathoexcitation does not elicit corresponding changes in vascular tone, suggesting diminished sympathetic signalling. Blunted sympathetic transduction following acute hypoxia, however, has not been confirmed and the effects of hypoxia on the sympathetic transduction of mean arterial pressure (MAP) as a function of action potential (AP) activity is unknown. We hypothesized that MAP changes would be blunted during acute hypoxia but restored in recovery and asynchronous APs would elicit smaller MAP changes compared to synchronous APs. Seven healthy males (age: 24 (3) yrs; BMI: 25 (3) kg/m2 ) underwent 20-min isocapnic hypoxia (PET O2 : 47 (2) mmHg) and 30-min recovery. Multi-unit microneurography (muscle sympathetic nerve activity; MSNA) and continuous wavelet transform with matched mother wavelet was used to detect sympathetic APs during baseline, hypoxia, early (first 7-min), and late recovery (last 7-min). AP groups were classified as synchronous APs, asynchronous APs (occurring outside a MSNA burst), and no AP activity. Sympathetic transduction of MAP was quantified using signal-averaging, with ΔMAP tracked following AP group cardiac cycles. Following synchronous APs, ΔMAP was reduced in hypoxia (+1.8 (0.9) mmHg) and early recovery (+1.5 (0.7) mmHg) compared to baseline (+3.1 (2.2) mmHg). AP group-by-condition interactions show that at rest asynchronous APs attenuate MAP reductions compared to no AP activity (-0.4 (1.1) vs. -2.2 (1.2) mmHg, respectively), with no difference between AP groups in hypoxia, early, or late recovery. Sympathetic transduction of MAP is blunted in hypoxia and early recovery. At rest, asynchronous sympathetic APs contributes to neural regulation of MAP by attenuating nadir pressure responses. Abstract figure legend Seven healthy men underwent 20-min isocapnic hypoxia and 30-min recovery. The study tested the hypotheses that hypoxia would blunt the sympathetic transduction of mean arterial pressure (MAP) following synchronous AP activity and that asynchronous APs would elicit smaller ΔMAP compared to synchronous APs. All sympathetic APs were detected and extracted from the filtered MSNA neurogram using a continuous wavelet transform with matched mother wavelet. AP groups were classified as synchronous (with MSNA burst), asynchronous (outside MSNA burst), and no AP activity. An effect of condition showed that following synchronous APs, ΔMAP was reduced in hypoxia and early recovery compared to baseline. AP group-by-condition interactions revealed that asynchronous APs attenuate MAP reductions compared to no AP activity under resting conditions. Our findings demonstrate that sympathetic transduction of MAP is blunted in hypoxia and remains diminished into early recovery. At rest, asynchronous AP activity contributes to MAP regulation by attenuating pressure reductions.
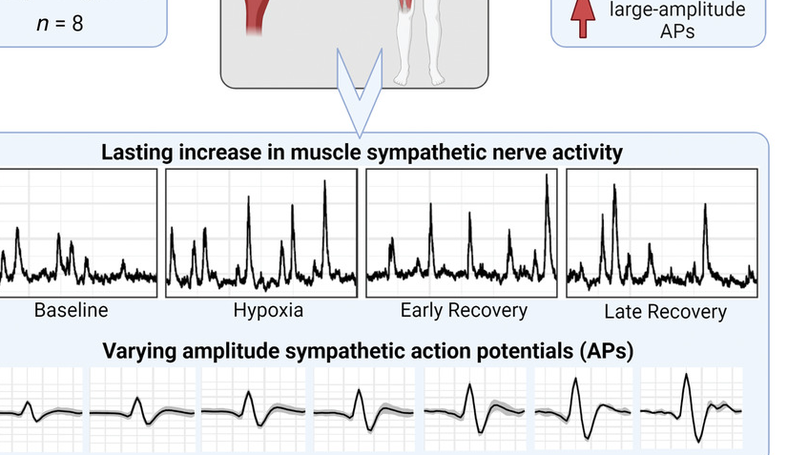
Baroreflex resetting permits sympathetic long-term facilitation (sLTF) following hypoxia; however, baroreflex control of action potential (AP) clusters and AP recruitment patterns facilitating sLTF is unknown. We hypothesized that baroreflex resetting of arterial pressure operating points (OPs) of AP clusters and recruitment of large-amplitude APs would mediate sLTF following hypoxia. Eight men (age: 24 (3) years; body mass index: 24 (3) kg/m2 ) underwent 20 min isocapnic hypoxia (PETO2: 47 (2) mmHg) and 30 min recovery. Multi-unit microneurography (muscle sympathetic nerve activity; MSNA) and a continuous wavelet transform with matched mother wavelet was used to detect sympathetic APs during baseline, hypoxia, early (first 5 min), and late recovery (last 5 min). AP amplitude (normalized to largest baseline AP amplitude), percentage APs occurring outside a MSNA burst (percentage asynchronous APs), and proportion of APs firing in small (1-3), medium (4-6) and large (7-10) normalized cluster sizes was calculated. Normalized clusters were used to assess baroreflex OPs and sensitivity. Hypoxia increased total MSNA activity, which remained elevated during recovery (P < 0.0001). Baroreflex OPs were shifted rightward for all clusters in recovery, with no effect on slope. Compared to baseline, AP amplitude was elevated by 3 (2)% and 4 (2)% while asynchronous APs were reduced by 9 (5)% and 7 (6)% in early and late recovery, respectively. In early recovery, the proportion of APs firing in large clusters was increased compared to baseline. Hypoxia-induced sLTF is mediated by baroreflex resetting of AP clusters to higher OPs, reduced asynchronous AP firing, and increased contribution from large-amplitude APs. KEY POINTS: Acute isocapnic hypoxia resets the arterial baroreflex and permits long-lasting sympathoexcitation, termed sympathetic long-term facilitation. Our understanding of sympathetic long-term facilitation following hypoxia in humans is based on multiunit muscle sympathetic nerve activity and does not fully characterize the underlying baroreflex control of sympathetic neuronal subpopulations or their discharge/recruitment strategies. We show that sympathetic long-term facilitation is mediated by baroreflex resetting of sympathetic action potential clusters to higher arterial pressure operating points, a reduction in the percentage of action potentials firing asynchronously, and a shift toward larger amplitude action potential activity. The results advance our fundamental understanding of how the sympathetic nervous system mediates sympathetic long-term facilitation following exposure to acute isocapnic hypoxia in humans.