Abstract
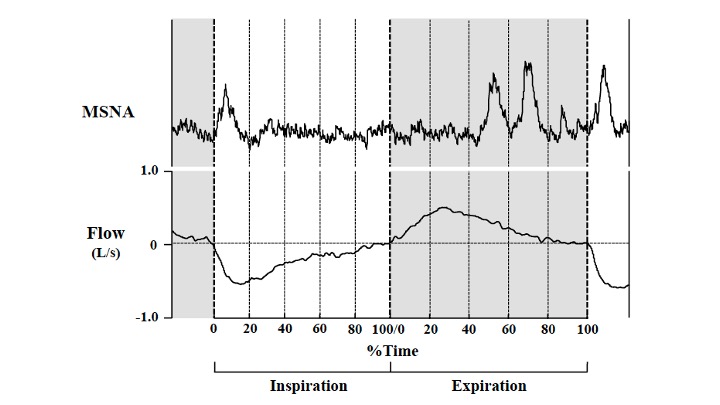
Respiratory modulation of sympathetic vasomotor outflow to skeletal muscles (muscle sympathetic nerve activity; MSNA) occurs in resting humans. Specifically, MSNA is highest at end-expiration and lowest at end-inspiration during quiet, resting breathing. We tested the hypothesis that within-breath modulation of MSNA would be amplified during graded leg cycling. Thirteen (n = 3 female) healthy young (age 25.2 ± 4.7 years) individuals completed all testing. MSNA (right median nerve) was measured at rest (baseline) and during semi-recumbent cycle exercise at 40%, 60%, and 80% of maximal workload (Wmax). MSNA burst frequency (BF) was 20.0 ± 4.0 bursts/min at baseline and was not different during exercise at 40%Wmax (21.3 ± 3.7 bursts/min; P = 0.292). Thereafter, MSNA BF increased significantly compared with baseline (60%Wmax: 31.6 ± 5.8 bursts/min; P textless 0.001, 80%Wmax: 44.7 ± 5.3 bursts/min; P textless 0.001). At baseline and all exercise intensities, MSNA BF was lowest at end-inspiration and greatest at mid-to-end expiration. The within-breath change in MSNA BF (DMSNA BF; end-expiration minus end-inspiration) was gradually increased from baseline to 60%Wmax leg cycling, but no further increase appeared at 80%Wmax exercise. Our results indicate that within-breath modulation of MSNA is amplified from baseline to moderate intensity during dynamic exercise in young healthy individuals, and that no further potentiation occurs at higher exercise intensities. Our findings provide an important extension of our understanding of respiratory influences on sympathetic vasomotor control.